The rapidly growing global e-mobility industry requires new, innovative flame retardants and demand keeps increasing massively
Compared to classic cars with combustion engines,
more
ECHA Suggestion of classification, labelling and also restriction of selected chlorinated flame retardants paves the way for an increased need for halogen free flame retardants
The European Chemicals Agency ECHA recently propos
more
The Working Group „Flame Retardants“ brings together many participants from the flame retardants value chain and celebrates Prof. Manfred Döring’s well-deserved retirement
Finally, the meeting of the working group “F
more
“ECOFRAM" addresses the need for more sustainable flame retardants and showcases developments from science and industry
The International Conference on Eco-Friendly Flame
more
RoHS: Impact study finds positive results, review process has started
The importance of RoHS, the restriction of hazardo
more
“Fire Resistance in Plastics" addresses the need for flame retardants for e-mobility – halogen-free solutions in clear focus
The Fire Resistance in Plastics is one of the most
more
Intumescent flame retardant systems

Mode of action: formation of a voluminous, insulating protective layer through carbonization and simultaneous foaming
Intumescent systems puff up to produce foams. They are used to protect combustible materials such as plastics or wood, and those like steel, which lose their strength when exposed to high temperatures, against the attack of heat and fire.
Basically, intumescent flame retardant systems consist of the following:
1. "Carbon" donors (e.g. polyalcohols such as starch, pentaerythritol)
2. Acid donors (e.g. ammonium polyphosphate)
3. Spumific compounds (e.g. melamine)
Process of intumescent mechanism
1. Softening of the binder/polymer (e.g. polypropylene)
2. Release of an inorganic acid (e.g. ammonium polyphosphate)
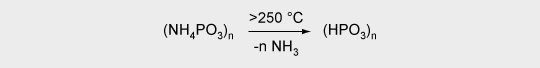
3. Carbonization (e.g. of polyalcohols)

4. Gas formation by the spumific compound (e.g. melamine)
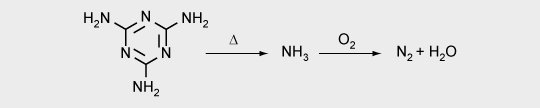
5. Foaming of the mixture
6. Solidification through cross-linking reactions
The picture below shows how the foam looks in the end. This coating expanded from a 1 mm layer to a 100 mm foam.
