03/Jan/2023
The rapidly growing global e-mobility industry requires new, innovative flame retardants and demand keeps increasing massively
Compared to classic cars with combustion engines,
more
The rapidly growing global e-mobility industry requires new, innovative flame retardants and demand keeps increasing massively
Compared to classic cars with combustion engines,
more
28/Nov/2022
ECHA Suggestion of classification, labelling and also restriction of selected chlorinated flame retardants paves the way for an increased need for halogen free flame retardants
The European Chemicals Agency ECHA recently propos
more
ECHA Suggestion of classification, labelling and also restriction of selected chlorinated flame retardants paves the way for an increased need for halogen free flame retardants
The European Chemicals Agency ECHA recently propos
more
07/Sep/2022
The Working Group „Flame Retardants“ brings together many participants from the flame retardants value chain and celebrates Prof. Manfred Döring’s well-deserved retirement
Finally, the meeting of the working group “F
more
The Working Group „Flame Retardants“ brings together many participants from the flame retardants value chain and celebrates Prof. Manfred Döring’s well-deserved retirement
Finally, the meeting of the working group “F
more
12/Jul/2022
“ECOFRAM" addresses the need for more sustainable flame retardants and showcases developments from science and industry
The International Conference on Eco-Friendly Flame
more
“ECOFRAM" addresses the need for more sustainable flame retardants and showcases developments from science and industry
The International Conference on Eco-Friendly Flame
more
22/Apr/2022
RoHS: Impact study finds positive results, review process has started
The importance of RoHS, the restriction of hazardo
more
RoHS: Impact study finds positive results, review process has started
The importance of RoHS, the restriction of hazardo
more
06/Jan/2022
“Fire Resistance in Plastics" addresses the need for flame retardants for e-mobility – halogen-free solutions in clear focus
The Fire Resistance in Plastics is one of the most
more
“Fire Resistance in Plastics" addresses the need for flame retardants for e-mobility – halogen-free solutions in clear focus
The Fire Resistance in Plastics is one of the most
more
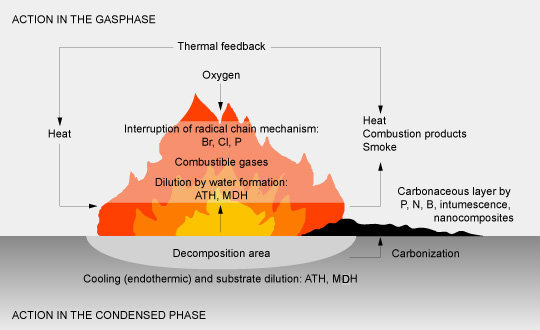
Mode of action
Flame retardants may slow down or even interrupt the combustion process by physical or chemical action in the solid, liquid or gas phase. They interfere during heating, decomposition, ignition or flame spread with the course of the fire. The most important processes are:
- Physical action by cooling (endothermic process of FR decomposition) or diluting the substrate in the gas phase (i.e. formation of water) and the solid phase (alumina trihydrate and magnesium hydroxide), or by coating the substrate (shielding it against the attack of oxygen and heat) with phosphorous and nitrogen compounds.
- Chemical action in the gas phase interferes with the combustion processes by eliminating the high energy H and OH radicals by halogen halides from halogenated flame retardants, metal halogen compounds from antimony trioxide, and phosphorous-containing fragments from phosphorous flame retardants (“flame poisoning”).
In the solid phase, the flame retardant forms a carbonaceous layer on the surface of the polymer by dehydration, formation of double bonds, thus initiating cyclization and cross-linking (phosphorous, nitrogen compounds, intumescent systems).